Medical Device Software Verification Validation And Compliance Pdf
Medical device software verification validation and compliance david a. Nancy knettell founder and principal at software510k llc has over 30 years in combined mechanical design software development and systems engineering experience primarily in the medical device industry as a software verification validationsystems engineer.
 Download Medical Device Software Verification Validation And
Download Medical Device Software Verification Validation And
medical device software verification validation and compliance pdf is important information accompanied by photo and HD pictures sourced from all websites in the world. Download this image for free in High-Definition resolution the choice "download button" below. If you do not find the exact resolution you are looking for, then go for a native or higher resolution.
Don't forget to bookmark medical device software verification validation and compliance pdf using Ctrl + D (PC) or Command + D (macos). If you are using mobile phone, you could also use menu drawer from browser. Whether it's Windows, Mac, iOs or Android, you will be able to download the images using download button.
It helps you think critically about software validation to build confidence in.
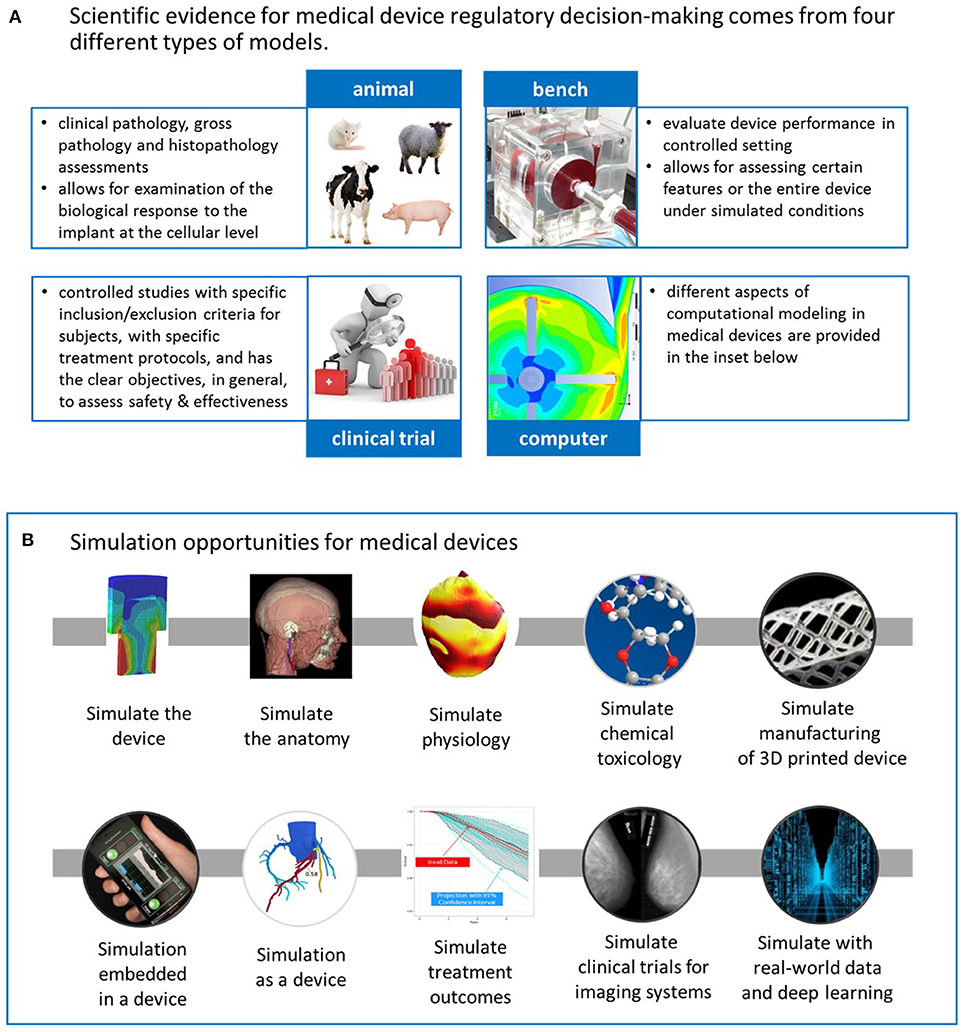
Medical device software verification validation and compliance pdf. This just published book is written specifically to help medical device and software engineers qa and compliance professionals and corporate business managers better understand and implement critical verification and validation processes for medical device software. The article also provides an overview of the ce marking application and 510k submission requirements for medical devices containing software. Of software validation medical device software non medical device software fda regulations and guidance.
What they are what they are not. Acknowledgments xxi background 1 chapter 1 the evolution of medical device software validation and the need for this book 3 the evolution of validation in the medical device. This book is written in a refreshingly dis tinctive manner to help readers better understand and implement critical verification and validation processes for medical device software.
Validating medical device software can be challenging especially when the software is a component part of or is embedded within a more complex medical device. Not only is it necessary to develop and test the software but it must be validated in accordance with regulatory expectations often with reference to the broader medical device. This article defines software verification and validation vv for medical devices.
Verifying the device design. This is a print on demand reproduction of the original title and does not include any dvd here s the first book written specifically to help medical device and software engineers. Define medical device software verification and validation vv posted by nancy knettell on december 13 2015.
Compliance officers would also benefit from this very practical book which deals with real time solutions to rather multifarious inputs and outputs. Vogel artech house boston. Design verification shall confirm that the design output meets the design input requirements.
Free shipping on qualifying offers. Heres the first book written specifically to help medical device and software engineers qa and compliance professionals and corporate business managers better understand and implement critical verification and validation processes for medical device software. This guidance outlines general validation principles that the food and drug administration fda considers to be applicable to the validation of medical device software or the validation of software used to design develop or manufacture medical devices.
Fda regulations medical device software regulated under 21 cfr 830 design controls. Medical device software verification validation and compliance david a.
 Medical Device Software Verification Validation And
Medical Device Software Verification Validation And
 Medical Device Software Verification Validation And Compliance
Medical Device Software Verification Validation And Compliance
 Download Medical Device Software Verification Validation And
Download Medical Device Software Verification Validation And
Pdf Medical Device Software Verification Validation And
Pdf Download Medical Device Software Verification Validation And Compliance Pdf Full Ebook
 Sofia Diaz Medical Device Software Verification Validation
Sofia Diaz Medical Device Software Verification Validation
 Fda Computer System Software Validation What You Ve
Fda Computer System Software Validation What You Ve
%2C445%2C291%2C400%2C400%2Carial%2C12%2C4%2C0%2C0%2C5_SCLZZZZZZZ_.jpg) Medical Device Software Verification Validation And
Medical Device Software Verification Validation And
 Medical Device Software Verification Validation And Compliance Pdf Online
Medical Device Software Verification Validation And Compliance Pdf Online
 What Is Software As A Medical Device Samd
What Is Software As A Medical Device Samd
Connected Device System Validation Quality Best