Software Validation Procedure Iso 13485 Template
Is the creation of a quality procedure. Requirements of iso 13485.
 Software Validation Procedure Iso 13485 Fda Qsr Compliant
Software Validation Procedure Iso 13485 Fda Qsr Compliant
software validation procedure iso 13485 template is free HD wallpaper was upload by Admin. Download this image for free in HD resolution the choice "download button" below. If you do not find the exact resolution you are looking for, then go for a native or higher resolution.
Don't forget to bookmark software validation procedure iso 13485 template using Ctrl + D (PC) or Command + D (macos). If you are using mobile phone, you could also use menu drawer from browser. Whether it's Windows, Mac, iOs or Android, you will be able to download the images using download button.
Validation of the application of computer software used.
Software validation procedure iso 13485 template. Specific approach and activities associated with software validation and. The software validation procedure governs computer systems and medical device software used in medical device development production and qa activities. What are iso 134852016.
The validation requirements of iso 13485 are specific to. The video provided below explains what is included when you purchase our standard operating procedures sops for iso 134852016. This procedure applies to all software whose use is.
This blog post discusses the inclusion of new requirements for quality system software validation in iso 13485. The documentation template may be used for iso 13485. Imsxpress iso 13485 template documentation is part of imsxpress iso 13485 software.
Record of software validation. Seven questions answered on iso 13485 validation. Use our free iso 13485 procedure template and the list of.
Procedure for documentation and validation. We continue this series on validation of software used in production and qms with. Iso 13485 document template.
Validation of software. This article remains relevant with the new requirements on software validation found in iso 134852016. The template documentation covers both iso 134852003 and fda qsr 21 cfr part 820.
The validation report template. Servicing procedure 754 validation.
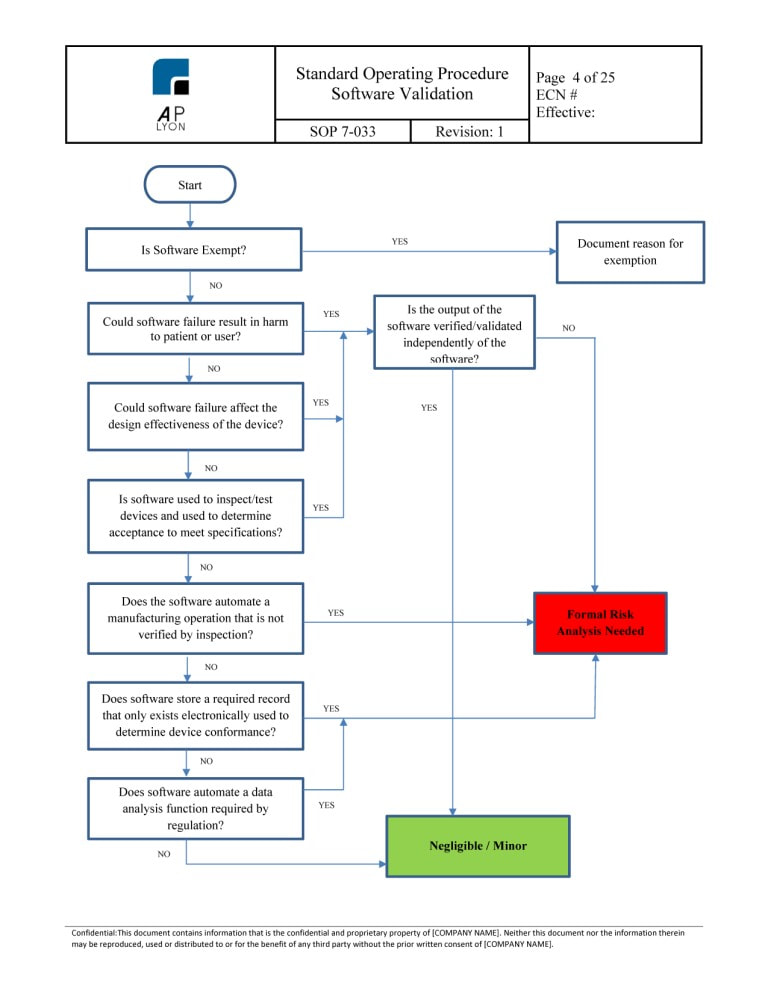 Software Validation Procedure Iso 13485 Fda Qsr Compliant
Software Validation Procedure Iso 13485 Fda Qsr Compliant
 Gamma Irradiation Sterilization Validation Procedure Iso 13485 Fda Qsr Compliant
Gamma Irradiation Sterilization Validation Procedure Iso 13485 Fda Qsr Compliant
 Validation Protocol And Report Procedure Iso 13485 Fda Qsr Compliant
Validation Protocol And Report Procedure Iso 13485 Fda Qsr Compliant
 Iso 13485 2016 Documentation Toolkit
Iso 13485 2016 Documentation Toolkit
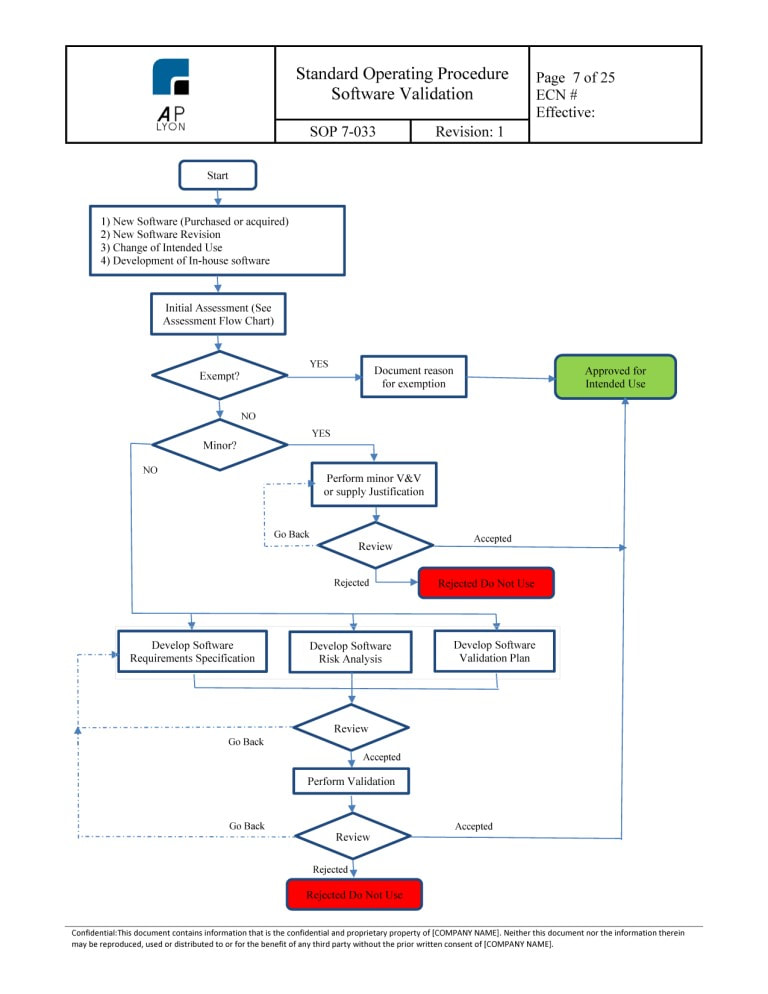 Software Validation Procedure Iso 13485 Fda Qsr Compliant
Software Validation Procedure Iso 13485 Fda Qsr Compliant
 Best Tips Iso 13485 Procedures With Our Free Template
Best Tips Iso 13485 Procedures With Our Free Template
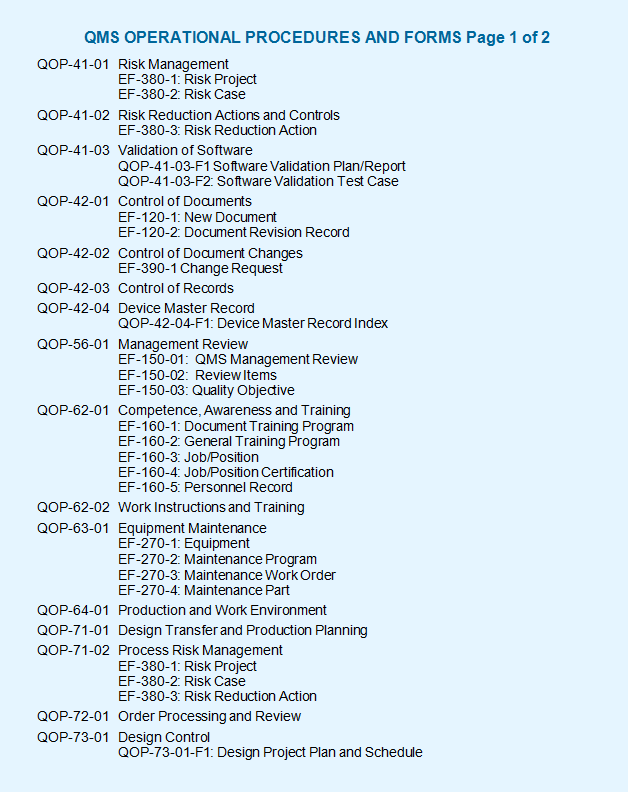 Imsxpress Iso 13485 Template Documentation Qms Management
Imsxpress Iso 13485 Template Documentation Qms Management
 Understanding The New Requirements For Qms Software
Understanding The New Requirements For Qms Software
Quality System Software Validation In The Medical Device
0 Response to "Software Validation Procedure Iso 13485 Template"
Post a Comment